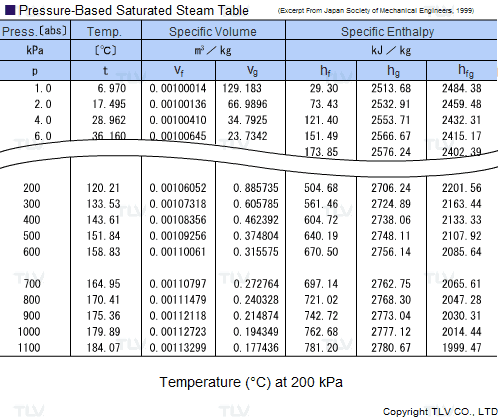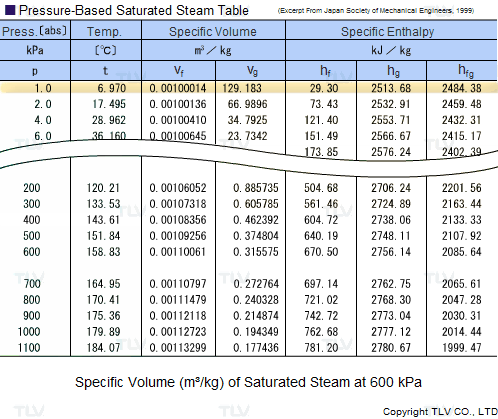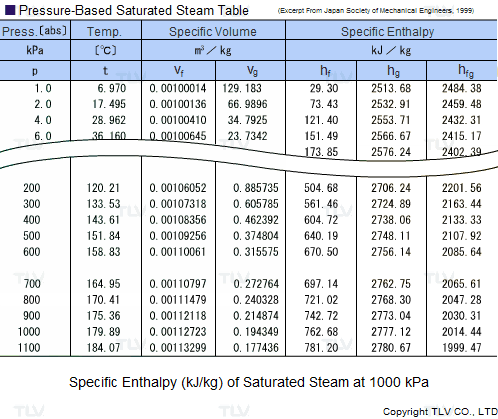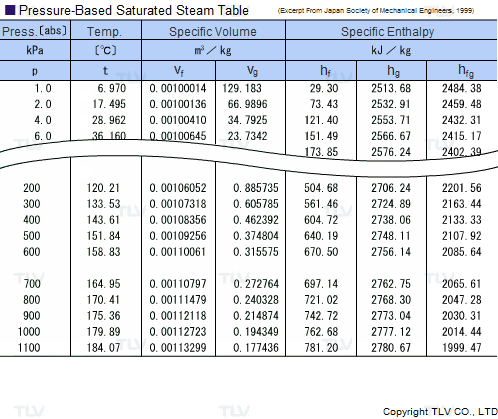
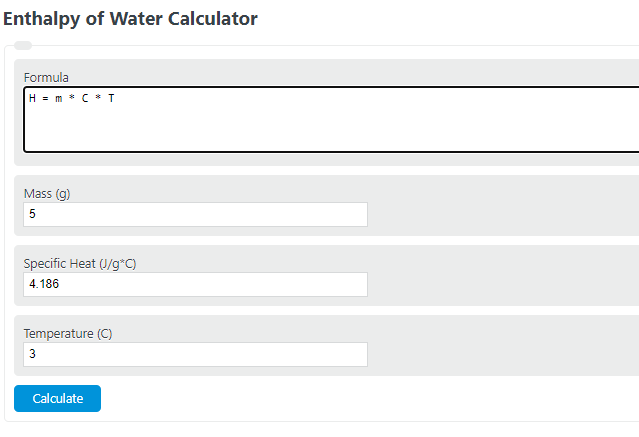
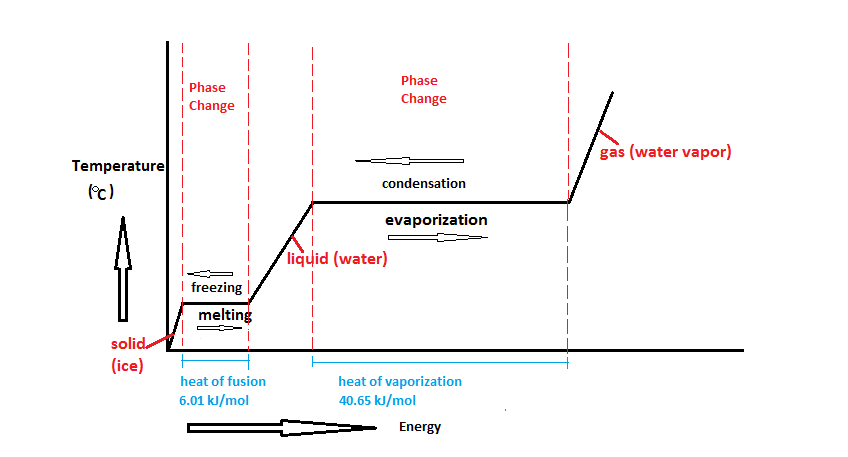
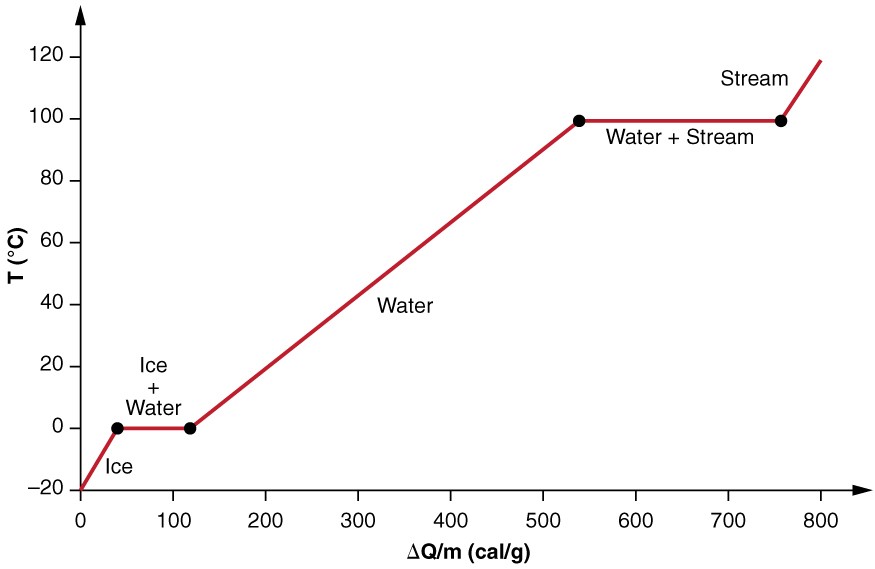
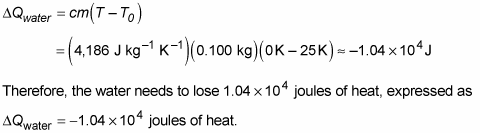Calculate the molal elevation constant of water if molar enthalpy of vaporisation of water at 373 K is 40.585 kJ/mol.

Calculate the enthalpy of vaporisation per mole for ethanol. Given, Δ S = 109.8JK^-1mol^-1 and boiling point of ethanol is 78.5^oC .

Using Heat of Fusion or Vaporization to Find the Heat Needed to Melt or Boil a Substance | Chemistry | Study.com

The latent heat of vaporisation of water is `9700 \"Cal/mole\"` and if the b.p.is `100^(@)C`, - YouTube